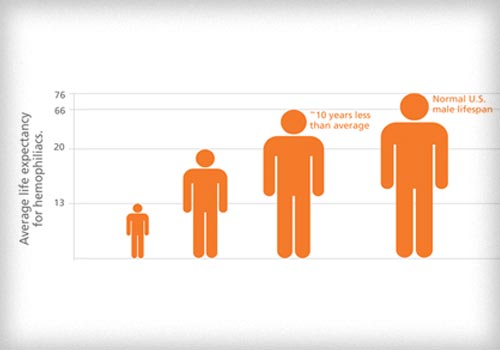
In terms of human and financial costs, few diseases rival the toll exacted by hemophilia, an inherited bleeding disorder. Briefly, the blood of hemophilia patients does not contain adequate amounts of the specialized proteins needed for blood clotting. As a result, they are subject to medical complications including anemia, internal bleeding, damage to muscles and joints and excessive bleeding after minor injuries.1
Hemophilia is relatively rare, with an estimated 20,000 Americans impacted, the overwhelming majority of whom are male.2 Hemophilia is also subdivided into two main types: hemophilia A and hemophilia B. Fortunately, a broad array of medications are now available for hemophilia patients.
However, treating hemophilia is extraordinarily expensive, with much of the overall cost linked to the price of the medicines themselves. The cost of drug therapy for a person with hemophilia can be several hundred thousand dollars per year and annual treatment costs of $1 million or more are not unheard of for patients with the most severe forms of the disease.3
Hemophilia treatment regimens vary according to the type of hemophilia (A or B) and severity of the disease. Most of the 28 drugs currently approved for hemophilia are known as replacement clotting factors. These drugs are injected into the body to replace the natural clotting proteins missing in hemophilia patients. In some cases concentrated clotting factors are used as on-demand treatments for bleeding incidents. However, increasingly the standard of care is to use clotting factors prophylactically, to prevent bleeding before it occurs.4
New and future treatments
Fortunately for hemophilia patients, new treatments options continue to emerge.
One longstanding issue for hemophilia patients is the need to frequently readminister clotting factor. Accordingly, many new versions of clotting factor are formulated to offer a longer half-life of efficacy. Approved in August 2018, Jivi® (antihemophilic factor [recombinant] PEGylated-aucl), promises a half-life of 17.9 hours, allowing for a longer interval between injections.5
One of the largest cost variables in treating hemophilia is whether or not the patient develops inhibitors – an immune reaction to the factor itself. One recently approved drug, Hemlibra® (emicizumab-kxwh), looks to sidestep the issue of inhibitors altogether. Instead of replicating a specific clotting factor, the drug works to bond existing clotting factors together to help form blood clots.6
Looking ahead, gene therapies that may eliminate the need for ongoing injections are currently in clinical trials. Hemophilia A and B are natural targets for gene therapies. In theory, if you replace the faulty gene responsible for hemophilia, you could free patients from a lifetime of injections.7
If the few gene therapies currently on the market in other classes are any indication, we can expect that any future gene therapies for hemophilia will also be very expensive. For example, Luxturna®, a gene therapy for a rare form of hereditary blindness, is priced to reflect the lifetime cost of a person’s untreated visual impairment.8 By that yardstick, an effective gene therapy for hemophilia could well exceed $1 million.
OptumRx takes a holistic approach to ensure better patient outcomes and lower costs related to treating hemophilia. OptumRx also closely monitors and evaluates the hemophilia drug development pipeline landscape for upcoming drug approvals.
Related content
References
- Centers for Disease Control and Prevention. “Complications of Hemophilia.” Accessed at: https://www.cdc.gov/ncbddd/hemophilia/facts.html
- National Hemophilia Foundation. “Fast Facts.” Accessed at: https://www.hemophilia.org/About-Us/Fast-Facts
- National Public Radio. “Miracle of Hemophilia drugs comes at a steep price.” Accessed: https://www.npr.org/sections/health-shots/2018/03/05/589469361/miracle-of-hemophilia-drugs-comes-at-a-steep-price
- Therapeutic Advances in Hematology. “New treatments in hemophilia: insights for the clinician.” Accessed at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3573440/
- U.S. Food and Drug Administration. “Package Insert – Jivi.” Accessed at: https://www.fda.gov/downloads/BiologicsBloodVaccines/UCM618979.pdf
- U.S. Food and Drug Administration. “FDA approves new treatment to prevent bleeding in certain patients with hemophilia A.” Accessed at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585567.htm
- Therapeutic Advances in Hematology. “Gene therapy for hemophilia: what does the future hold?” Accessed at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6130099/
- OptumRx. “The impending age of million-dollar drugs” Accessed at: https://www.optum.com/resources/library/million-dollar-drugs.html
STATEMENT REGARDING FINANCIAL INFLUENCE:
This article is directed solely to its intended audience about important developments affecting the pharmacy benefits business. It is not intended to promote the use of any drug mentioned in the article and neither the author nor OptumRx has accepted any form of compensation for the preparation or distribution of this article.